
It remains unclear if this holds true for the different ASCO/CAP FISH amplified groups. In addition, some clinical trials showed that pCR rates are lower in oestrogen receptor (ER)-positive/HER2-positive BC than in ER-negative/HER2-positive BC. However, little attention has been paid to predictors of pCR among the different ASCO/CAP FISH groups.
#Pcr complete response plus#
Retrospective studies of HER2-positive BC patients who have received NACT plus neoadjuvant anti-HER2 therapy have reported a higher rate of pCR in IHC 3+ compared to IHC 2+/ HER2 amplified tumours. The most widely-agreed definition of pCR is no residual invasive carcinoma both in the breast and axillary lymph nodes regardless of the presence of residual ductal carcinoma in situ (DCIS) (ypT0/is ypN0). The pathological complete response (pCR) rate at the time of surgery is commonly used as an endpoint in clinical trials and a predictor of good prognosis in HER2-positive BC with neoadjuvant treatment.

įor HER2-positive BC, defined as either IHC 3+ or IHC 2+ with HER2 amplification, neoadjuvant chemotherapy (NACT) plus neoadjuvant anti-HER2 therapy is an effective treatment option. The 2018 ASCO/CAP guidelines divide HER2 FISH status into five groups (Table 1). If the IHC result is score 2+, such patients are tested for HER2 amplification by ISH most commonly fluorescence in situ hybridisation (FISH), to assess the average HER2 gene and chromosome enumeration probe 17 (CEP17) copy numbers (CNs) per carcinoma cell and the ratio of these. The current American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines regard HER2 IHC score 3+ as positive, score 2+ as equivocal and scores 0 and 1+ as negative. Immunohistochemistry (IHC) and/or in situ hybridisation (ISH) is routinely used to evaluate the HER2 status for treatment selection. Currently, a combination of sequential chemotherapy and anti-HER2 therapy is the standard treatment for HER2-positive BC both in the neoadjuvant and adjuvant setting. Repeat HER2 testing after neoadjuvant treatment should therefore be considered.Īpproximately 15% of invasive breast cancers (BCs) are human epidermal growth factor receptor2 (HER2) positive, defined as showing HER2 gene amplification or protein overexpression, and such tumours have been shown to be sensitive to anti-HER2 targeted therapy. In the current study, 22% of HER2-positive tumours became HER2-negative by IHC and FISH following neoadjuvant treatment, the majority (74%) HER2 IHC 2+/ HER2 amplified tumours. In the HER2 IHC 2+/ HER2 amplified tumours or ASCO/CAP FISH Group 1 alone, ER-negativity was an independent predictor of pCR following NACT and/or neoadjuvant anti-HER2 therapy. Multivariate logistic regression analysis showed that HER2 IHC 3+ and histological grade 3 were independent predictors of pCR following neoadjuvant anti-HER2 therapy. Pathological complete response (pCR) was more frequent in HER2 IHC 3+ tumours than in HER2 IHC 2+/ HER2 amplified tumours, when either in receipt of NACT alone (38% versus 13% p = 0.22) or neoadjuvant anti-HER2 therapy (52% versus 20% p < 0.001). HER2 IHC 2+ tumours were classified into five groups by fluorescence in situ hybridisation (FISH) according to the 2018 ASCO/CAP guidelines of which Groups 1, 2 and 3 were considered HER2 amplified.
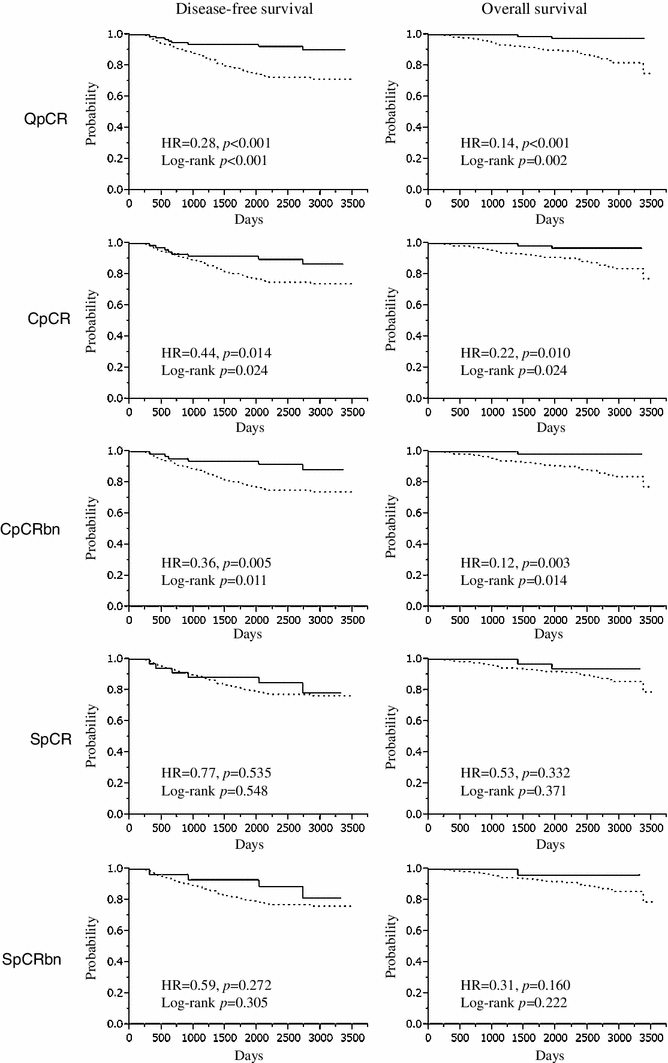
The study group comprised 500 BC patients treated with neoadjuvant chemotherapy (NACT) and/or neoadjuvant anti-HER2 therapy and surgery who had tumours that were 3+ or 2+ with HER2 immunohistochemistry (IHC). This study aims to identify predictors of response in the neoadjuvant treatment and to assess the discordance rate of HER2 status between pre- and post-treatment specimens in HER2-positive BC patients. However, the response is not uniform and a proportion of HER2-positive patients do not respond. The response of human epidermal growth factor receptor2 (HER2)- positive breast cancer (BC) patients to anti-HER2 targeted therapy is significant.


 0 kommentar(er)
0 kommentar(er)
